By Shantanu Mukherjee and Anushka Iyer
RECAP OF PART 1
The use of wearable devices capable of identifying key human biomarkers (e.g. blood pressure and oxygen levels) sharply accelerated during the Covid-19 pandemic. The deadly coronavirus came as an unpleasant reminder of the human body’s frailty and triggered a desire among many to lead healthier lives, leading to increased consumer demand for general wellness aids and fitness trackers. In the healthcare ecosystem, physicians and healthcare institutions, now physically removed from their patients, began to use these devices to remotely monitor those at risk.
Today, when your watch can track your heartbeat and oxygen levels, the line between patient and consumer, and fitness wearable and medical device, has blurred. Can these devices be accommodated within existing regulatory structures, or do they deserve sui generis treatment? In Fitness Wearables and the Law: Part I, we looked at how fitness wearables are regulated in the United States, including the FDA’s guidance on the regulation of wearables intended for general wellness purposes.
Here, in Part II, we navigate the regulatory and legal trends in respect of fitness wearables in the European Union.
BACKGROUND
Until recently, there were three primary regulations (“Prior Legislation”) governing medical devices in the EU:
i) Directive 90/385/EEC on active implantable medical devices;
ii) Directive 93/42/EEC on medical devices (“MDD”); and
iii) Directive 98/79/EC on in vitro diagnostic medical devices.
The European Commission felt the need, just as the US FDA had, to address the growing prominence of technology-driven health products. Towards that end, the European Commission on 25 May 2017, simultaneously enacted two major regulatory changes that replaced the Prior Legislation:
i) EU Regulation 2017/745 on medical devices (“MDR”); and
ii) EU Regulation 2017/746 on in vitro diagnostic medical devices (“IVDR”).[i]
Although the MDR was enacted in 2017, the EU provided for a three-year transition period for the MDR to come into effect. This transition period was later extended due to the impact of the Covid-19 pandemic on healthcare and medical device stakeholders,[ii] and the MDR finally came into effect on 26 May 2021, replacing the existing MDD and the directive on active implantable medical devices. The MDR introduces new concepts, definitions, and rules which may be made applicable to technology-driven health products, some of which are discussed and analyzed below.
ARE SMART WEARABLES CONSIDERED MEDICAL DEVICES UNDER THE MDR?
What are smart wearables?
Although the term ‘smart wearables’ is not defined anywhere in any of the EU directives, the European Commission[iii] in an informational paper refers to it as “a body-borne computational and sensory devices which can sense the person who wears them and/or their environment. Wearables can communicate either directly through embedded wireless connectivity or through another device (e.g. a smartphone). The data collected by the wearable device about the user or its environment is processed in a processing unit located locally or in an external server, and the results are ultimately provided to the wearer. Smart wearables may have control, communication, storage and actuation capabilities.”
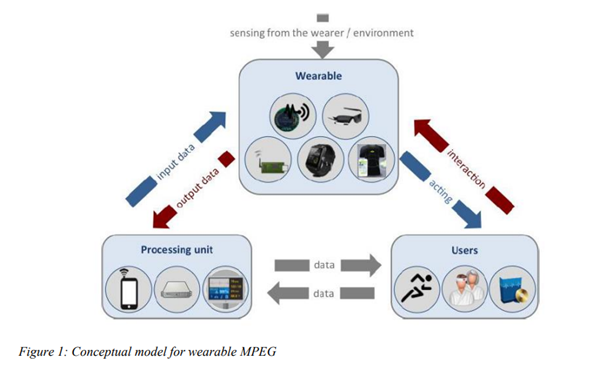
The paper also provides examples of such wearables, which include blood pressure monitors, ECG monitors, hearing aids, smartwatches, smart glasses, sleep sensors, and the like.
INTENDED USE OF A MEDICAL DEVICE
To understand how the current framework of the MDR differs from the MDD, let’s look at how medical devices are defined under both regimes.
Article 1 (2) of the MDD defines a medical device as “any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of” (among other things) “diagnosis, prevention, monitoring, treatment or alleviation of disease…” or for the “…investigation, replacement or modification of the anatomy or of a physiological process…”[iv]
From the above, it is clear that under the MDD, a medical device may include software, and that the manufacturer’s intended use for the device is key to determining the device’s nature. The intended purpose is defined as “the use for which a device is intended according to the data supplied by the manufacturer on the label, in the instructions for use or in promotional or sales materials or statements and as specified by the manufacturer in the clinical evaluation.”[v]
Article 2(1) of the MDR defines a medical device as “any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
– diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease,…
– …providing information by means of in vitro examination of specimens derived from the human body, including organ, blood and tissue donations…”[vi]
The definitions above make it clear that under the MDD, if a manufacturer intends for its device to diagnose, prevent, monitor, treat, alleviate, or investigate a disease or a physiological process, then the device will be considered a medical device, and be subject to the MDD’s requirements.
The MDR, in its definition of a medical device, introduces the additional qualifying language: “prediction” and “prognosis”. Therefore, a fitness wearable that ‘predicts’ or provides a ‘prognosis’ of future disease indication, such as e.g. diabetes, through analysis of the data it collects, would arguably qualify as a medical device under the MDR (unlike under the erstwhile MDD), even if it does not diagnose, prevent or treat such disease.
The Guidance Document on the classification of medical devices[vii] bears out this reading. For instance, under the MDD, most standalone software used for diagnostic or medical purposes fell under Class I (lower-risk level) and therefore, was subject to fewer regulatory requirements. Under the MDR, most standalone software that falls within the definition of a medical device automatically falls under Class II A or higher.
Therefore, for manufacturers of smart wearables, the advent of the MDR may mean potentially increased regulatory compliance requirements, and the need to demonstrate general safety and performance before legally placing their product on the European market. As with the MDD, the intended use of the device remains key.[viii] The Preamble to the MDR also clarifies that “…software in its own right, when specifically intended by the manufacturer to be used for one or more of the medical purposes set out in the definition of a medical device, qualifies as a medical device, while software for general purposes, even when used in a healthcare setting, or software intended for life-style and well-being purposes is not a medical device.”[ix]
Smart wearables that are intended for “lifestyle and well-being purposes” therefore, appear exempt. Arguably, a smartwatch capable of reading and monitoring the wearer’s heart rate may not qualify as a medical device when it is intended to be used for general fitness purposes but could qualify as a medical device if its stated purpose is to monitor or prevent cardiac disease.[x] Manufacturers would do well to keep this distinction in mind when designing their device packaging, product inserts, promotional materials, and their warranties and terms of use. For instance, the Garmin smartwatch, which is capable of monitoring heartbeat, sleep, stress, activity, and oxygen levels is not registered as a medical device and is disclaimed for use for medical purposes as reflected in the disclaimers and marketing materials.[xi]
The European Court of Justice (“ECJ”), in the case of Brain Products v. Biosemi,[xii] took a similar view and held that “[where] a product is not conceived by its manufacturer to be used for medical purposes, its certification as a medical device cannot be required”. Therefore, how the manufacturer of a device advertises the product has an important bearing on the classification of a smart wearable as a medical device,[xiii] which in other words, can be interpreted to mean that how a device is actually used has no bearing on how the device is regulated if the manufacturer doesn’t intend for it to be a medical device.
INTENDED VERSUS ACTUAL USE
Similar to the US, if the function or actual use of the device may be medical, despite any disclaimers to that effect, such devices should ideally be registered under the MDR. For instance, the Samsung Galaxy Watch 4 allows for tracking sleep, activity, blood pressure, stress, and heart rate. Although the smartwatch is intended for fitness and wellness purposes, calculation, monitoring, and analysis of blood pressure through its app is registered as a medical device across the EU, UK, Singapore, and UAE.[xiv]
Although the concept of intended use is aimed to encourage and ease market entry for fitness and ‘holistic wellness’ wearables, there is increasing evidence that notwithstanding the stated intent of manufacturers, these wearables are sometimes used by consumers as the basis for medical decision-making.[xv] The MDR aims to address this by providing for post-market surveillance where manufacturers may voluntarily collect, and review information to understand how their products are being used by the public to better align regulatory purposes with public health objectives and identify any preventive measures, where necessary.
Part III of our Fitness Wearables and the Law series will examine the legal and regulatory framework for smart wearables in India, contrasting it to the US and EU regulatory frameworks.
[i] See Regulation 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/ 2009 and repealing Council Directives 90/385/EEC and 93/42/EEC [2017], art. 20, O.J. (L 117/1) (EU); nSee also Regulation 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU [2017], art. 18, O.J. (L 117/176) (EU).
[ii] See Commission postpones application of the Medical Devices Regulation to prioritize the fight against coronavirus, https://ec.europa.eu/commission/presscorner/detail/en/ip_20_589.
[iii] See Smart Wearables: Reflection and Orientation Paper, https://www.astrid-online.it/static/upload/smar/smartwearablesreflectionandorientationpaper.pdf.
[iv] COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01993L0042-20071011&from=EN.
[v] Article 2(12), REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices.
[vi] REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745.
[vii] MDCG 2021-24 Guidance on classification of medical devices, October 2021, https://ec.europa.eu/health/system/files/2021-10/mdcg_2021-24_en_0.pdf.
[viii] See, Id., The Guidance Document provides that while classifying a product as a medical device, the intended use and not the accidental use of the device will determines the class of the device. It is the intended purpose assigned by the manufacturer to the device that determines the class of the device and not the class assigned to other similar products.
[x] Are Wearables Medical Devices Requiring a CE-Mark in the EU?, Covington Digital Health, 22 Jan 2019, https://www.covingtondigitalhealth.com/2019/01/are-wearables-medical-devices-requiring-a-ce-mark-in-the-eu/.
[xi] Simple and streamlined. Garmin vivosmart 5 fitness tracker is the effortless way to take charge of your everyday health, Bloomberg, 20 April 2022, https://www.bloomberg.com/press-releases/2022-04-20/simple-and-streamlined-garmin-v-vosmart-5-fitness-tracker-is-the-effortless-way-to-take-charge-of-your-everyday-health.
[xii] Case C-219/11 Brain Products GmbH v. Biosemi VOF and Others.
[xiii] Are Wearables Medical Devices Requiring a CE-Mark in the EU?, Covington Digital Health, 22 Jan 2019, https://www.covingtondigitalhealth.com/2019/01/are-wearables-medical-devices-requiring-a-ce-mark-in-the-eu/.
[xiv] Disclaimers, Samsung, https://www.samsung.com/uk/watches/galaxy-watch/galaxy-watch4-black-bt-sm-r870nzkaeua/.
[xv] Helen Yu, Regulation of Digital Health Technologies in the European Union, Intended versus Actual Use, Cambridge University Press, https://www.cambridge.org/core/services/aop-cambridge-core/content/view/D1C211DD82EAEAD44511508B781F5C63/9781108838634c8_103-114.pdf/regulation-of-digital-health-technologies-in-the-european-union.pdf.